Mastering Design Control in the Medical Device Industry
Introduction
In the dynamic ecosystem of the medical device industry, maintaining a perfect blend of revolutionary innovation and rigorous regulation is a demanding but indispensable feat. This balance is significantly governed by an effective implementation of design controls—a cardinal part of product development that ensures safety, efficacy, and adherence to international standards. Among these standards, ISO 13485 distinguishes itself as a globally accepted quality management system (QMS) for medical device manufacturers.
Confronted with modern challenges and heightened global competition, the need for advanced tools to support ISO 13485 compliance has grown exponentially. The solution lies in leveraging the prowess of Electronic Quality Management Systems (eQMS), enriched with a dedicated Design Control module. This cutting-edge digital tool doesn’t merely facilitate ISO 13485 adherence—it empowers your organization to excel beyond these standards, setting a trajectory for your products that redefines industry benchmarks.
The Indispensable Role of Design Control
Design control refers to systematic procedures instituted to validate that a device’s design meets specific design inputs and conforms to all required regulatory norms. However, to comprehend the true significance of design control, we must delve deeper into its modus operandi and understand why it is of paramount importance in medical device manufacturing.
Design controls are essentially proactive measures woven into the fabric of the product lifecycle—initiating from the nascent stages of product conceptualization, traversing through design and development, culminating in production, and extending to all post-production activities.
The importance of design control goes beyond its regulatory mandate—it’s a strategic move, a golden thread that ties together risk management, device safety enhancement, performance optimization, and superior quality outcomes. Under the purview of ISO 13485, design control lays the foundation for a fluid product lifecycle and fosters a culture of consistent excellence, permeating every layer of the organization.
The Game-changing Advent of eQMS
The introduction of eQMS is akin to a seismic shift in the landscape of quality management, transforming it into a seamless, efficient, and compliance-driven process. This powerful system represents a paradigm shift from the traditional, paper-reliant methods of maintaining design controls, often associated with high labor intensity, susceptibility to errors, and inefficiency.
The dawn of eQMS marks the beginning of a new era, where quality management is infused with digital innovation. It offers a centralized, automated platform that revolutionizes the way manufacturers manage and document design controls. This shift is embodied in the Design Control module of Momentum QMS, a tool engineered specifically to ensure ISO 13485 compliance while pioneering your organization’s journey of continuous quality improvement.
eQMS: The Beacon of Competitive Advantage
A closer scrutiny of the Design Control module in Momentum QMS uncovers a plethora of advantages it brings to your organization. Ranging from optimizing operations and fostering collaboration, to effective risk management, simplifying regulatory compliance, and enhancing traceability and documentation—this module is your all-in-one toolkit for excelling in the realm of quality management in medical device manufacturing.
1. Amplifying Efficiency: Momentum QMS orchestrates a symphony of efficiency in your workflow. By automating repetitive tasks, it minimizes manual errors, conserves valuable man-hours, and accelerates decision-making processes. In an industry where every moment saved can translate into a life rescued, such efficiency gains are not merely beneficial—they are transformational.
2. Cultivating Collaboration: Momentum QMS serves as a platform for seamless collaboration across diverse teams. By enabling real-time access to critical data, it fosters transparency, leading to quicker issue resolution and smoother progress of design projects. This system promotes a culture of synergy, boosting collective output, and promoting organizational cohesion.
3. Fortifying Risk Management: The Design Control module of Momentum QMS adopts a proactive stance towards risk management. From the earliest stages of the design process, potential risks are identified, evaluated, and mitigated, substantially minimizing product hazards and ensuring adherence to the highest safety standards.
4. Streamlining Regulatory Compliance: In the medical device industry, compliance with regulations like ISO 13485 isn’t a luxury—it’s a necessity. Momentum QMS simplifies this regulatory maze, facilitating compliance by ensuring that all processes align with set standards and that documentation is accurately maintained.
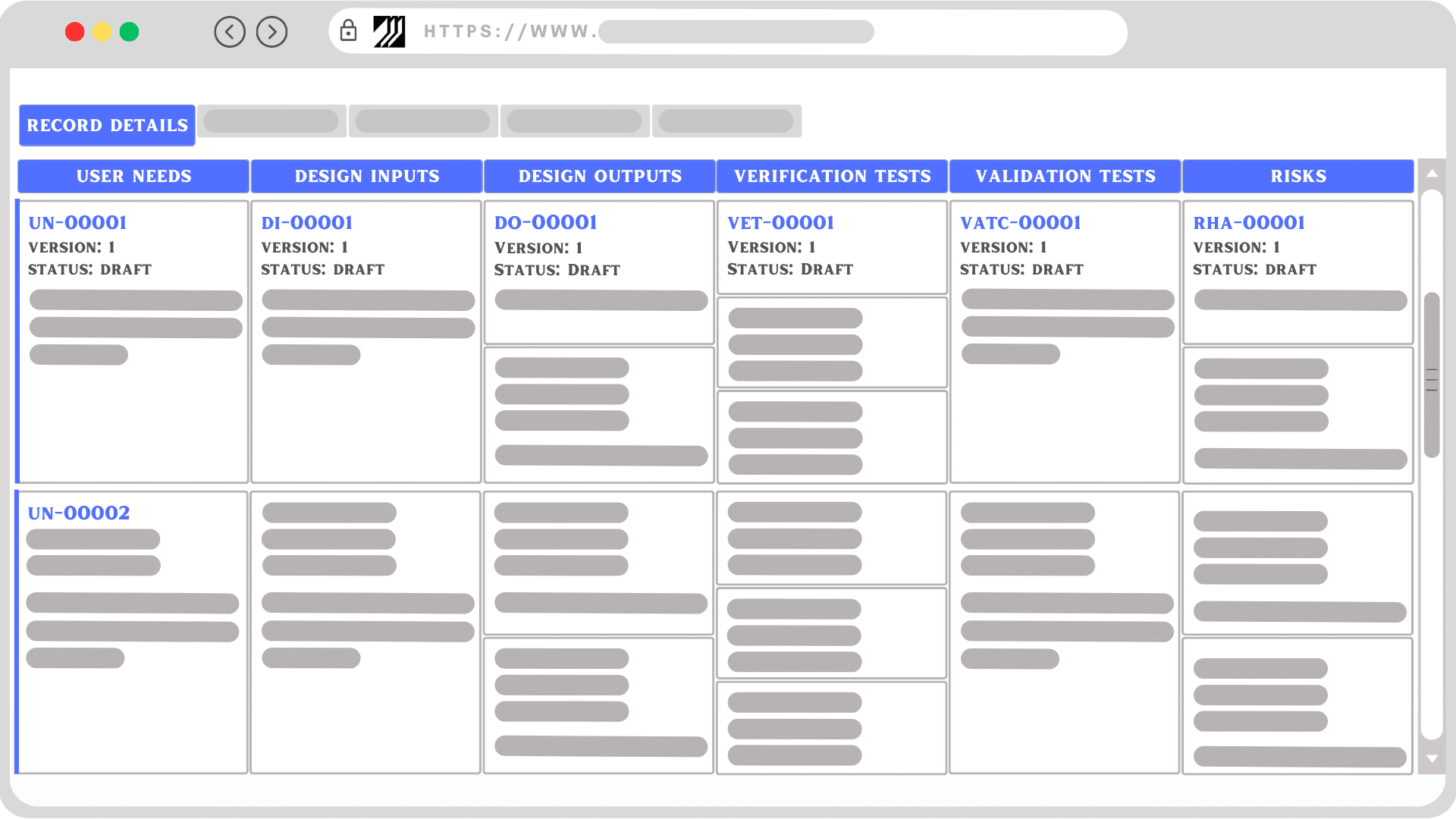
5. Enhancing Traceability and Documentation: In a regulated industry like medical devices, documentation and traceability are vital. The Design Control module helps manage all design-related data in a structured, searchable, and easily retrievable manner, offering complete visibility of the design and development process.
Deep Dive: Managing User Needs, Design Inputs/Outputs, Verification, Validation, and Risk with Momentum QMS
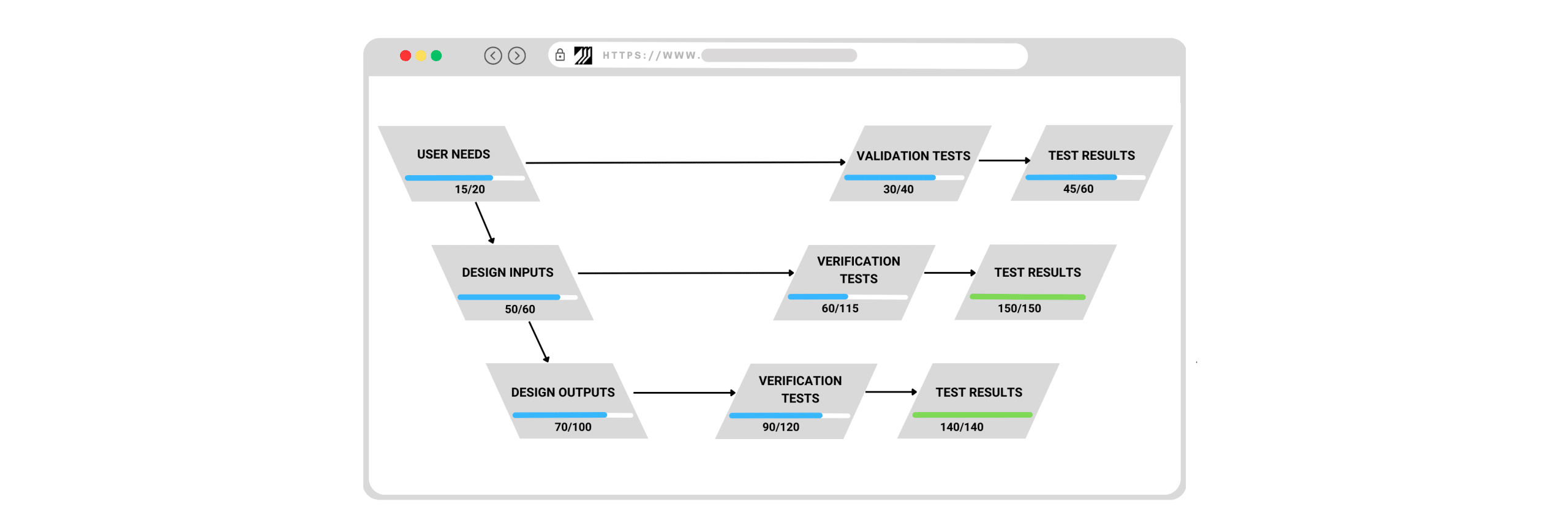
Momentum QMS’s Design Control module, ingeniously designed to support the various stages of the design and development process, assists in effectively managing User Needs, Design Inputs, Design Outputs, Verification, Validation, and Risk.
User Needs: At the core of successful medical device manufacturing are the user needs the product is designed to address. Momentum QMS enables you to capture user needs, keeping them as a reference throughout the design process. It encourages a collaborative approach, ensuring that all stakeholders understand these needs and align their efforts towards meeting them.
Design Inputs and Outputs: Design inputs define the characteristics that a device must possess to meet user needs and regulatory requirements. Momentum QMS ensures that these inputs are managed in a structured, traceable format. The system also makes sure that every design input is accounted for in subsequent design stages. As for design outputs, which are the tangible results of the design process, Momentum QMS offers real-time tracking, allowing for dynamic adjustments as the design evolves.
Verification and Validation Tests: Verification and validation tests are integral to confirming that a design meets its predetermined specifications and intended use. Design Control module streamlines these processes by offering tools to plan, execute, and document both verification and validation tests. The module also enables a clear record of test procedures, results, and any necessary corrective actions, ensuring that every design decision is evidence-based and traceable.
Risk Management: The Design Control module is adept at managing risk. By integrating with risk management tools, it supports comprehensive risk assessment and documentation. It also helps in conducting detailed risk assessments and maintains comprehensive risk documentation. It supports risk mitigation activities, ensuring they are implemented and tracked efficiently.
Conclusion: Embracing the Future of Quality Management with Momentum QMS
Integrating Momentum QMS into your design process signifies an investment in your organization’s future. It represents a strategic shift towards quality, efficiency, and success in a highly competitive landscape. Embrace the power of design control with Momentum QMS and lead the way in the ever-evolving medical device industry. Experience a paradigm shift in your approach to design control and quality management, and propel your organization to new heights of product excellence and regulatory compliance.