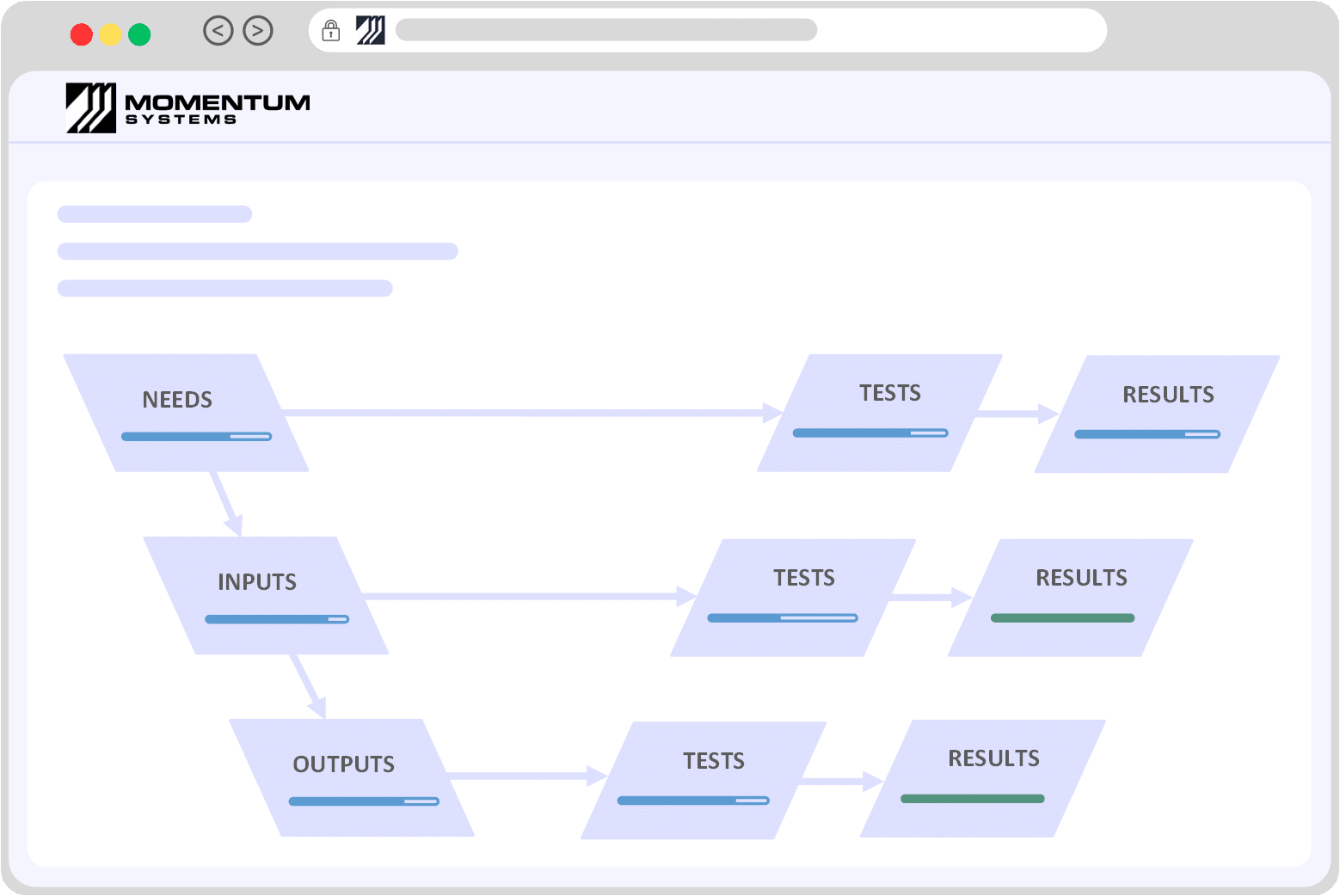
ISO 13485 SOFTWAREMedical Devices / ISO 13485 / Design Control Software
Navigate the intricacies of the medical device sector with Momentum QMS. Built on open-source technology, our software adeptly handles industry challenges and meets regulatory demands.
Ensure compliance without incurring user license fees. Streamline your processes and enhance your quality management with ease. Schedule a demo and explore the future of ISO 13485 compliance.
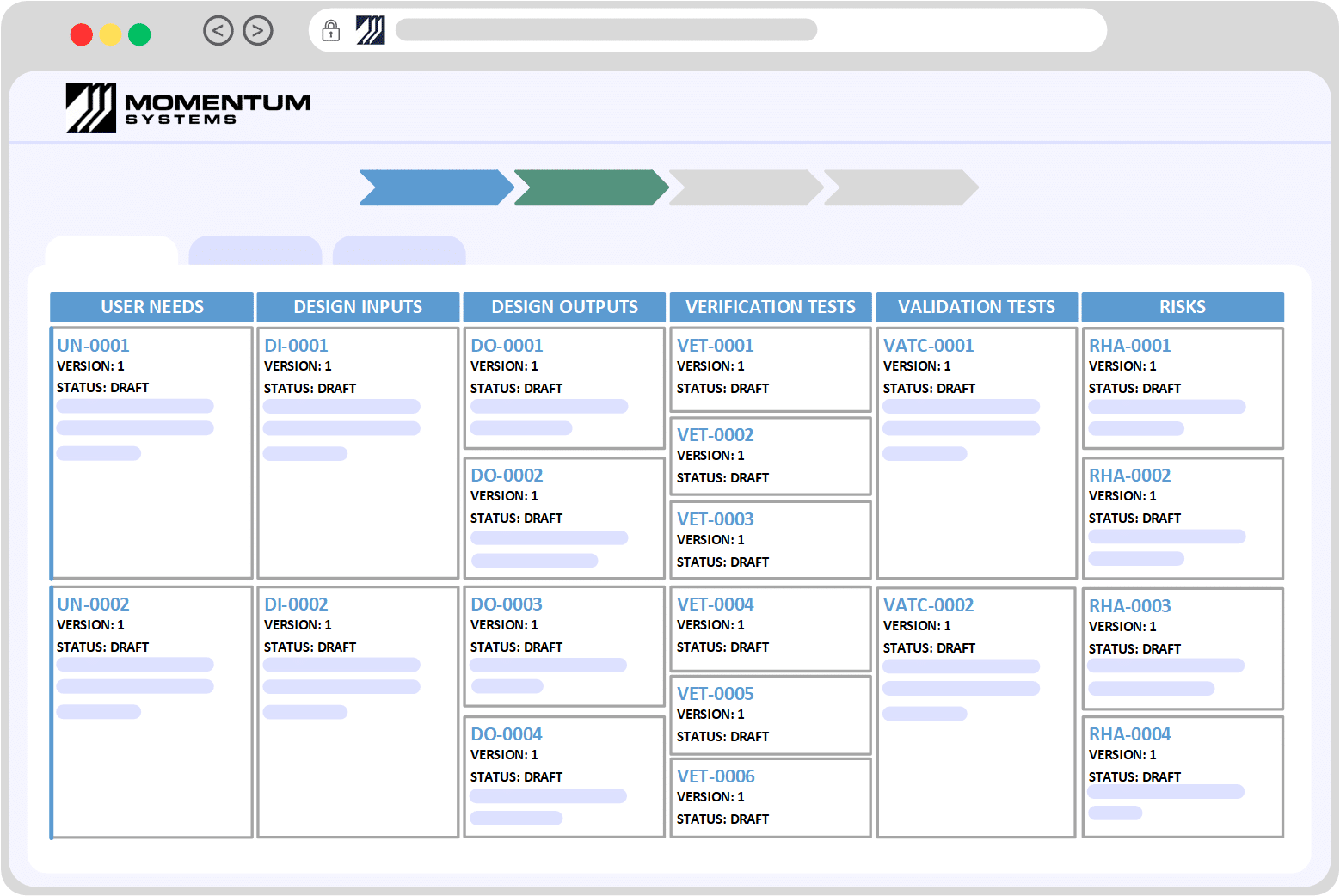
ISO 13485 Software – No User License FeePowered by OpenSource Technology, Crafted Without Compromise
The medical device industry grapples with a myriad of complex challenges, stemming from:
- Expanding product lines or geographical reach.
- Shifts in ownership or responsibility within the supply chain.
- Rising regulatory demands, including those like 21 CFR Part 11.
- Challenges in maintaining a thorough oversight of the supply chain.
- Reliance on manual processes and inadequately integrated IT systems.
- Cost management pressures.
Momentum QMS equips organizations with the essential tools and methodologies to navigate these hurdles. It aids in upholding an effective ISO 13485 compliant Quality Management System. Furthermore, Momentum QMS can be both validated and implemented in line with key regulatory standards such as 21 CFR Part 11.
Empower your decision-making and reach your aspiration of establishing and sustaining ISO 13485 compliance with Momentum QMS.
| ISO 13485: Section | Requirement | Momentum QMS |
|---|
| 4: General Requirements | Understanding the context and needs of the Organisation. Determine the scope and continually improving the QMS. Maintaining records and documentation related to the design, use and manufacture of devices. | Provides industry best practices and can be tuned to meet your organisation’s unique requirements/processes. Out of the box processes for managing and distributing documents and records. Key Modules/Takeaways: Flexibility and Adaptability, Document Control |
| 5: Management Responsibility Planning Customer Focus Management Review | Quality is not one person’s responsibility, leadership needs to be accountable and promote the principles of Quality Management. Risk-based thinking – Quality Objects and Planning | Decentralization of a centralized system. Momentum QMS is a centralized software however the roles, responsibilities and accountability is de-centralized. This allows leadership to not only communicate, enforce and promote Quality principles but at the same time enable teams to work collaboratively to solve problems. Out of the box modules Key Modules/Takeaways: Centralized Software, Customer Feedback, Meetings Management, Objectives Management |
| 6: Resource Management | Organization should demonstrate they have control over resources, including Human Resources, infrastructure and working environment. | Momentum QMS enables you to manage data related to customers, suppliers, equipment, assets etc. Moreover employee data including the management of training (offline or online) can be performed directly within the system. The system also comes with Health and Safety modules that enable you to manage the requirements related to the working environment. Key Modules/Takeaways: Master Data, Employee Training and Incident Management |
| 7: Product Realization | Requirements related to the planning, review, design, purchasing and creation of the product or service. | Momentum QMS comes with an array of integrated modules that enable organisations to manage the lifecycle of a product or service. Utilities such as Risk Assessments can be integrated at desired stages of the lifecycle to prioritize and add/justify controls Key Modules/Takeaways: Document Control, Product Specification Management, Risk Management, Integrated Modules |
| 8: Measurement, Analysis and Improvement | Monitoring of the QMS, assessing Customer Satisfaction, Internal Audits, managing Non Conformances and implementing corrective/preventive actions. | Using the Customer Feedback module customer relationship is enhanced and any improvements are carried out in a systemic manner. Audits can be developed and captured directly in the system with actions and investigations assigned to appropriate people so that there is accountability within the team. With standard modules for tracking Issues, Non-Conformances and Corrective actions you can be assured that all manner of issues are properly reported, assessed, investigated and controlled. Reporting, Trending and Alerts form a key component of the continuous monitoring, measurement and improvement of the system. Meetings management allow the capture of management reviews and any actions are tracked in the system for future followup meetings. Key Modules/Takeaways: Customer Feedback, Audits, Meetings and Analytics |
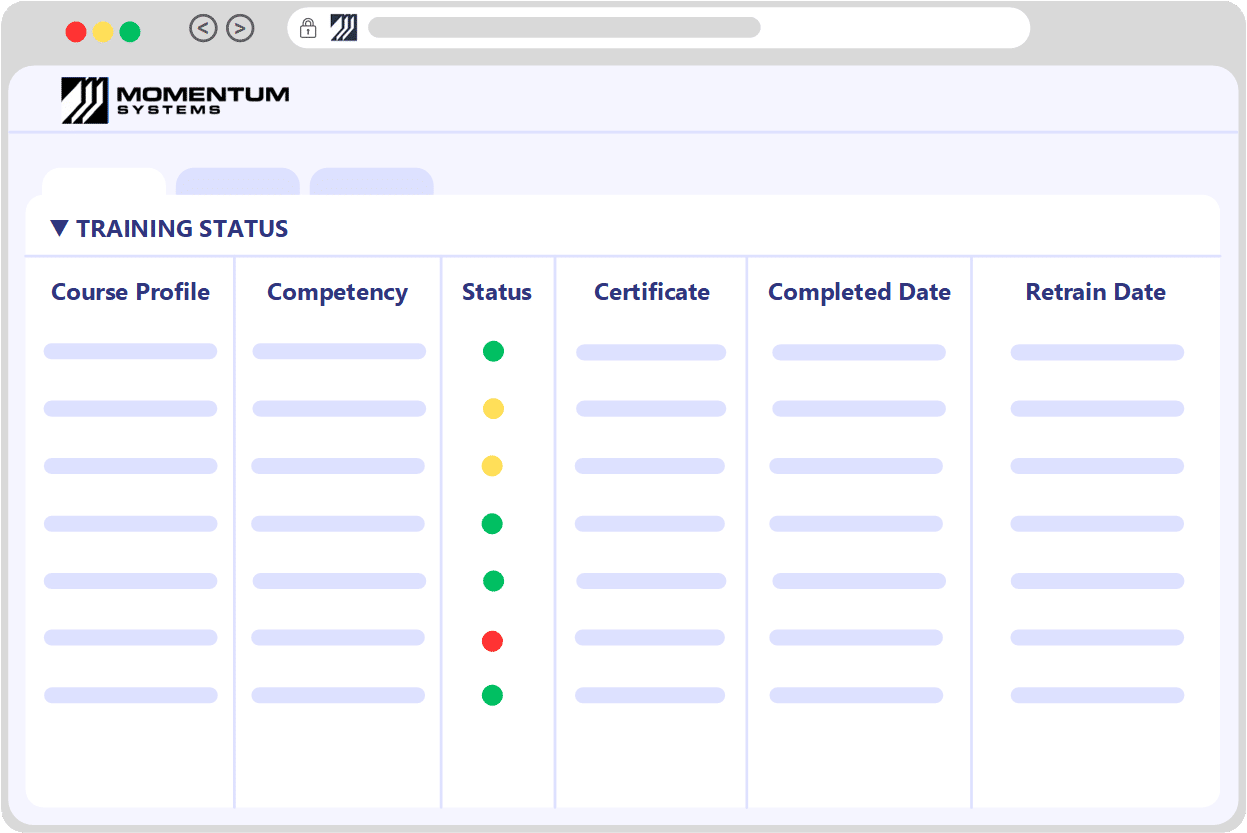
Training and Document ControlThe Foundation of Your Quality System: Training Matrix
Training and document control are fundamental pillars of any quality system. Momentum’s Training Management System meticulously logs employee training, ensuring you're always aware of imminent or upcoming sessions — a key factor in maintaining compliance.
While the Training module can function autonomously, it also seamlessly integrates with the Document Control module. This integration facilitates the tracking of various competencies directly associated with an employee. These competencies can either be evaluated within the system itself or recorded during on-site training courses.

Audits and Inspection ManagementOptimized for Mobile Access
Easily import your existing checklists into Momentum QMS. The platform facilitates capturing custom responses, taking photographs, appending electronic signatures, and more.
Efficiently raise findings and delegate tasks to both team members and external users. Schedule audits with confidence, ensuring that prior audit findings are diligently monitored and resolved.
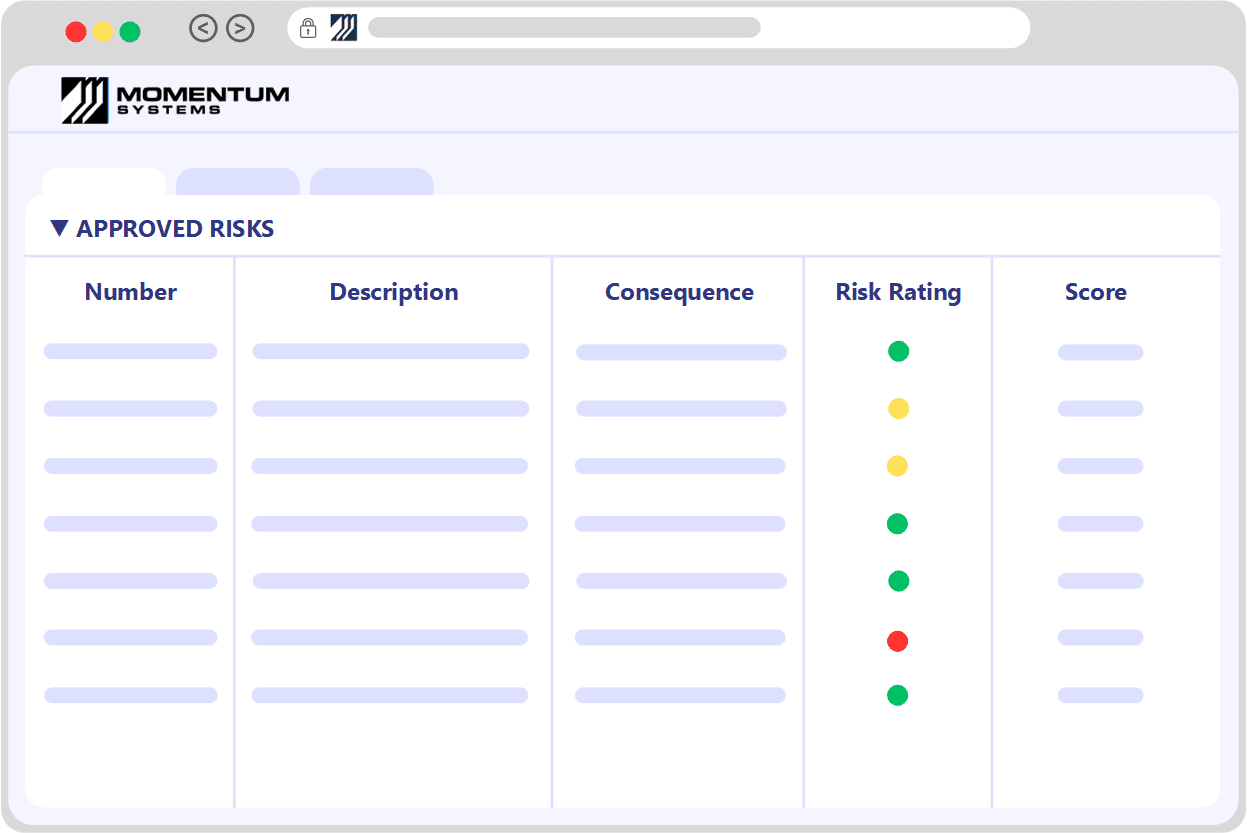
Risk RegisterIdentify, Assess, Control & Monitor
Seamlessly import your existing risk matrix and register, ensuring a swift start with your risk framework.
Potential risks may stem from various sources such as product issues, customer complaints, food safety/HACCP concerns, hazards, incidents, and more. Utilize a multifaceted risk matrix for assessments, incorporating numerous dimensions like severity, likelihood, and detectability. Our system offers fully configurable risk matrices, paired with comprehensive guidance to aid users in making well-informed decisions.
Assign the implementation of controls to team members, ensuring consistent monitoring until successful completion. Craft alerts and set up periodic checks to reassess risks, guaranteeing that critical concerns are never overlooked.
Discover the Full Potential of Momentum QMS20+ Modules or Customize to Fit Your Needs
Modify one of our existing modules or build your own modules from scratch. Automating manual processes has never been easier. All modules come with industry best practices and they are all included with no restrictions. The hardest decision you have to make is decide which module to start with.
- Aspects and Objectives
- Audits
- Calibration and Maintenance
- Change Management
- Corrective and Preventive Action
- Customer Feedback
- Delegation
- Document Control
- HACCP
- Health and Safety
- Deviations
- Job Safety
- Master Data
- Meeting Management
- Non-conforming Material
- Operations
- Product Specification Management
- Project Control
- Receiving and Inspections
- Risk Management
- Supplier Management
- Training and Learning Management
What Our Customers SaySee why clients around the world trust Momentum QMS to reduce costs and boost productivity, innovation, and competitiveness.

"The range of modules available is extensive. The existing modules and out-of-the-box functionality are easy to use and well designed. New modules can be created where required and existing modules adapted and customised to user needs."
Fiona M.
Quality Manager

"While many companies offer a software application to manage specific areas of a QMS I was looking for a "One Stop Shop" where I could manage all aspects of the quality system and utilize it to drive continuous improvement! Momentum QMS checked every box on the list (and then some)."
Ryan B.
Quality Manager
See why we are the most flexible and cost effective platform in the market today.













